Research
Neuro Technology Group
Neuro Technology group
Advancing brain science requires improved methods to read neural activity in the entire brain non-invasively and modulate neural activity at a localized site. Aim of the Neuro Technology group is to develop and refine fMRI approaches for high sensitivity and specificity, to develop devices for monitoring and modulating brain activity, to map functional networks with advanced fMRI methodologies, and to investigate neurovascular couplings in diseased models.
(1) Functional Neurovascular Mapping (Director Seong-Gi Kim, Collaborating with Yongho Kim)
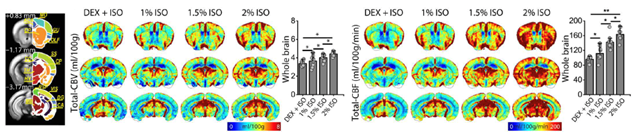
Commonly used BOLD fMRI approach is sensitive to baseline blood volume and oxygen level changes. To compare BOLD responses across different brain regions and status, it is critical to measure baseline CBV and BOLD fMRI. Neuro Technology group developed whole-brain BOLD-dynamic susceptibility contrast (DSC) MRI in response to transient hypoxia for determing baseline CBV and CBF. This method proved to be highly sensitive, repeatable within each imaging session, and across four weekly sessions. Relative cerebral blood volumes measured by BOLD DSC agree well with those by contrast agents. Quantitative cerebral blood volume and flow metrics were successfully measured in mice under dexmedetomidine and various isoflurane doses using both total vasculature-sensitive gradient-echo and microvasculature-sensitive spin-echo BOLD MRI. Dexmedetomidine reduces cerebral perfusion, while isoflurane increases cerebral perfusion in a dose-dependent manner.
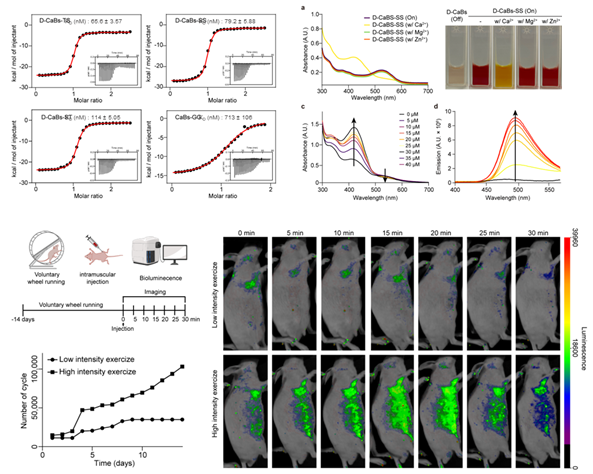
Also, we focused on the structural optimization of a photoswitchable calcium indicator and its application in in vivo imaging. Using Rosetta’s backrub method and synthetic amino acid substitution, we optimized the favorability for calcium binding, constructing a mutated indicator with D-amino acids. Candidates were selected based on RMSD (Root Mean Square Deviation) and RMSF (Root Mean Square Fluctuation) values, and the binding affinity was validated using isothermal titration calorimetry (ITC), confirming selectivity, photoswitchability, and dimer formation. The indicator's effectiveness was demonstrated in biological environments, with fluorescence intensity varying based on exercise intensity. Importantly, the insights gained from this work led to the development of a calcium indicator with improved selectivity and binding affinity, enabling calcium sensing in biological environments, thereby allowing the measurement of neuronal transmission. We developed molecular delivery strategies capable of penetrating key biological barriers such as the brain, skin, and cell membranes. This effort centered on two main approaches: the design of barrier-permeable peptides and the development of structurally stabilized, protein-based imaging probes. In this work, we utilized screening in combination with cheminformatics and sequence clustering to identify peptides. Furthermore, our study demonstrates how structure-guided SaCas9 engineering can enhance the in vivo functionality of protein-based tools. This strategy can be extended to the development of structurally stable protein-based imaging probes.
(2) Neural Interfaces (Collaborating with Donghee Son and Mikyung Shin)
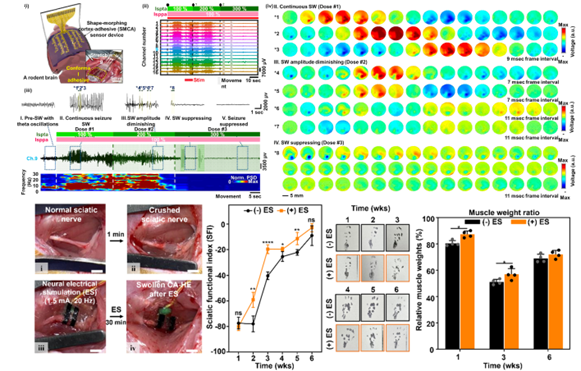
We developed an innovative shape-morphing cortex-adhesive (SMCA) sensor designed for closed-loop transcranial focused ultrasound (tFUS) neurostimulation. This sensor integrates catechol-conjugated alginate hydrogel adhesives with viscoplastic self-healing polymer substrates, forming a highly conformal and robust interface with cortical surfaces. Unlike conventional rigid or less-adaptive electrodes, this approach ensures artifact-resistant recording of neural signals during ultrasound exposure, effectively capturing high-frequency oscillations indicative of imminent seizures. Consequently, the SMCA sensor enables precise real-time feedback for modulating ultrasound therapy, demonstrating remarkable efficacy in seizure suppression within awake rodent models. This technology represents a major advancement in patient-specific, minimally invasive neurotherapy for conditions such as drug-resistant epilepsy.
SELECTED PUBLICATIONS
- Le et al., (2024) Mapping cerebral perfusion in mice under various anesthesia levels using highly sensitive BOLD MRI with transient hypoxia. Science Advances, 10, eadm7605
- Le et al., (2025). Whole-brain BOLD responses to graded hypoxic challenges at 7 T, 9.4 T, and 15.2 T: Implications for ultrahigh-field functional and dynamic susceptibility contrast MRI. Magnetic Resonance in Medicine. 94(1). 262-277
- Bae et al., (2024) Peptide‐drug conjugate with statistically designed transcellular peptide for psoriasis‐like inflammation. Advanced Healthcare Materials, 13(15):e2303480
- Kang et al., (2024) Structure-guided engineering of thermodynamically enhanced SaCas9 for improved gene suppression. Advanced Materials, 2404680
- Lee et al., (2024) A shape-morphing cortex-adhesive sensor for closed-loop transcranial ultrasound neurostimulation. Nature Electronics, 7:800
- Seong et al., (2024). Sticky and strain-gradient artificial epineurium for sutureless nerve repair in rodents and nonhuman primates. Advanced Materials, 36(16). 2307810

